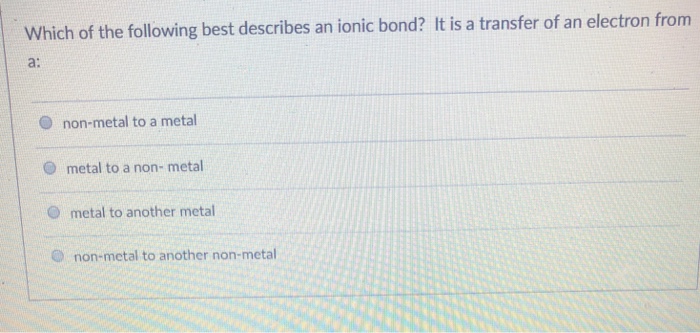
Which of the Following Best Describes an Ionic Bond
Formed by the sharing of electrons between a metal and a nonmetal. A force that holds two oppositely charged ions together.

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
Ionic bonds are formed between a cation which is usually a metal and an anion which is usually a nonmetal.

. The sharing of a hydrogen atom between two other atoms a relatively weak bond. It is a measure of the strength of. Which of the following best describes what happens when an ionic bond forms.
PO43-Which statement is true about crystal lattice energy. Which of the following would have an electrostatic force of attraction. Formed by the transfer of electrons between a metal and a nonmetal.
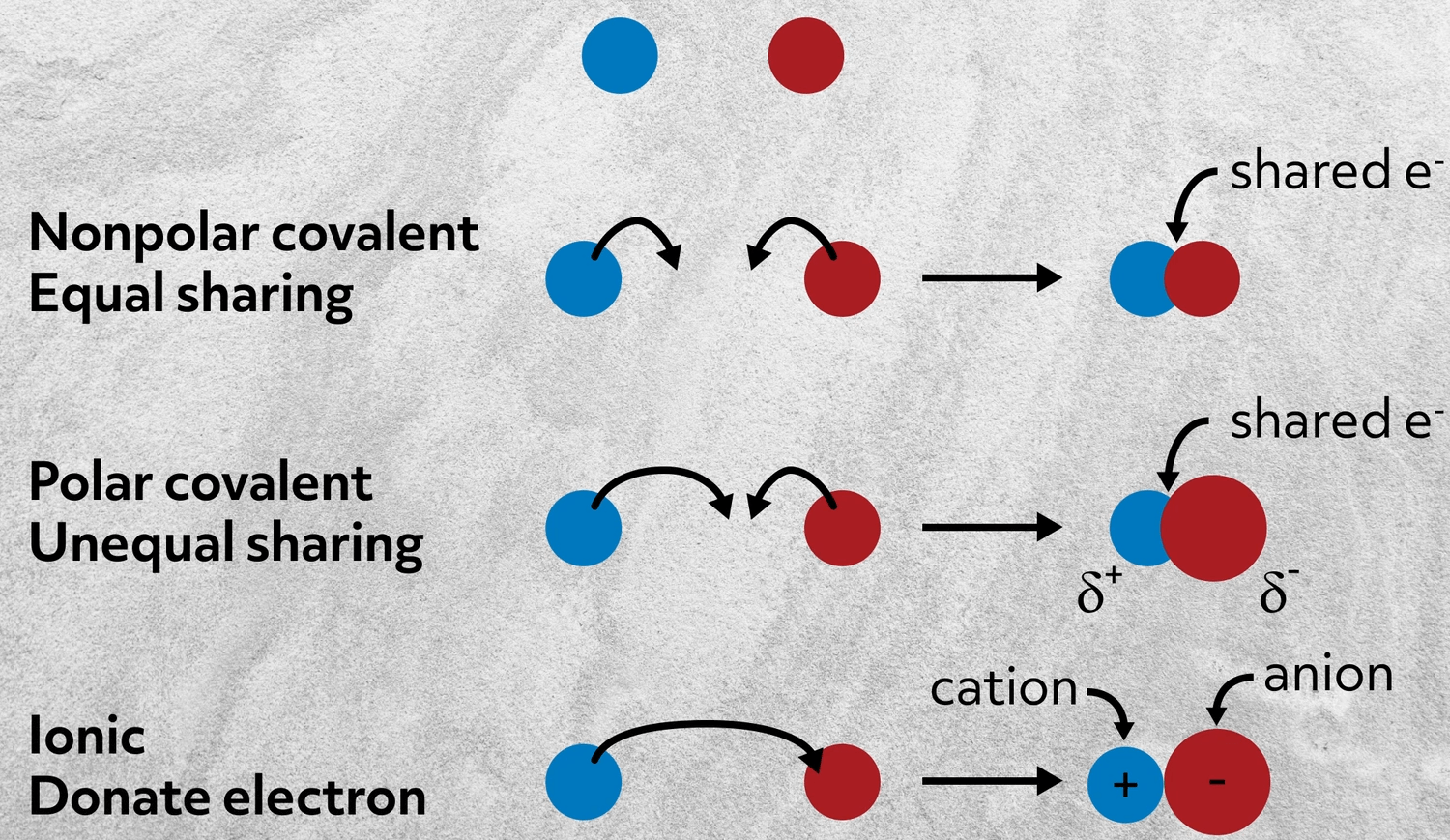
A covalent bond involves a pair of electrons being shared between atoms. A two atoms come together and share electrons in order to fill their octets. Just think of positive and negative charges attracting to one another thats really all an ionic bond is.
The answer is d An ionic bond involves a metal that transfers one or more electrons to a nonmetal. Rather than forming molecules or being measured by molecular weight as in choices A and B ionic compounds form large arrays of ions in crystalline solids and are measured with formula weights. Which chemical species can easily form an ionic bond with a cation.
C two atoms one atom which is more electronegative than the other exchange electrons and the charges hold the atoms together. Ionic bonding is the complete transfer of. It is called an ionic bond because when an element gives up or accepts electrons it gains either a positive or negative charge respectively.
Which of the following pairs of elements would most likely form a salt. Just think of positive and negative charges attracting to one another thats really all an ionic. Ionic bonds form only between metals and nonmetals.
The distribution of electrons between atoms. An ionic bond is the force of attraction that holds together positive and negative ions. Which of the following best describes a pair of elements that will form an ionic bond.
Which of the following best describes an ionic bond. Which of these best describes an ionic bond. An ionic bond involves a metal that transfers one or more electrons to a non-metal.
In ionic bonds electrons are not really shared but rather are donated from the less. Which of the following describes an ionic bond. It is called an ionic bond because when an element gives up or accepts electrons it gains either a positive or negative charge respectively.
The bond between two polyatomic ions. Hydrogen easily loses electrons and carbon gains them. Ionic bonding also commonly called electrovalent bonding is a type of chemical bonding that involves the bonding formed by the electrostatic attraction between atoms with a high electronegative difference as seen in group 1 atoms and group 7 atoms.
Ionic bonds always take place between a metal and nonmetal. View the full answer. The sharing of a pair of electrons between two atoms a relatively strong bondb.
What happens in the process of ionic bonding. What is the most likely formula unit of this salt. Option C is correct.
Metals usually give up electrons and nonmetals usually accept electrons. A found in most compounds in organisms B involves sharing of electrons C involves gaining or losing neutrons D involves gaining or losing electrons A saturated lipid contains a A more oxygen atoms than hydrogen atoms B no double or triple bonds in the fatty acids chains C. The attraction between two charged atoms a relatively weak bond in an aqueoussolution.
Formed by the sharing of electrons between two nonmetals. C the attraction that holds the atoms together in a polyatomic ion. E a bond between a metal and a polyatomic ion.
Group of answer choices. It takes energy to remove valence electrons from an atom and form a positive ion. What molecules form ionic bonds.
Ionic bonds associate charged particles with large differences in electronegativity. The sharing of a pair of electrons between two atoms a relatively weak bond. 10Which of the following statements best describes an ionic bond.
An ionic bond is best described as a attraction between 2 nonmetal atoms b the transfer of electrons from one atom to another. Which of the following situations best describes an ionic bond. B two atoms give up their electrons in order to form a bond.
Thats because metals want to give up electrons and nonmetals want to gain electrons. A salt forms in the reaction of barium with chlorine. Formed by the transfer of electrons between a nonmetal and a nonmetal.
Metals usually give up electrons and nonmetals usually accept electrons. Ionic bonds always take place between a metal and nonmetal.

Ionic Bond Definition Properties Examples Facts Britannica

Which Of These Best Describes An Ionic Bond Brainly Com
Solved Which Of The Following Best Describes An Ionic Bond Chegg Com
No comments for "Which of the Following Best Describes an Ionic Bond"
Post a Comment